Figure 51
Biological Clocks in Mosquitoes - Section 2
|
From the available data, such as the results used for Tables 1 and
2, it seems likely that for most mosquitoes, with their bimodal
activity, the clocks act side by side rather than being summative.
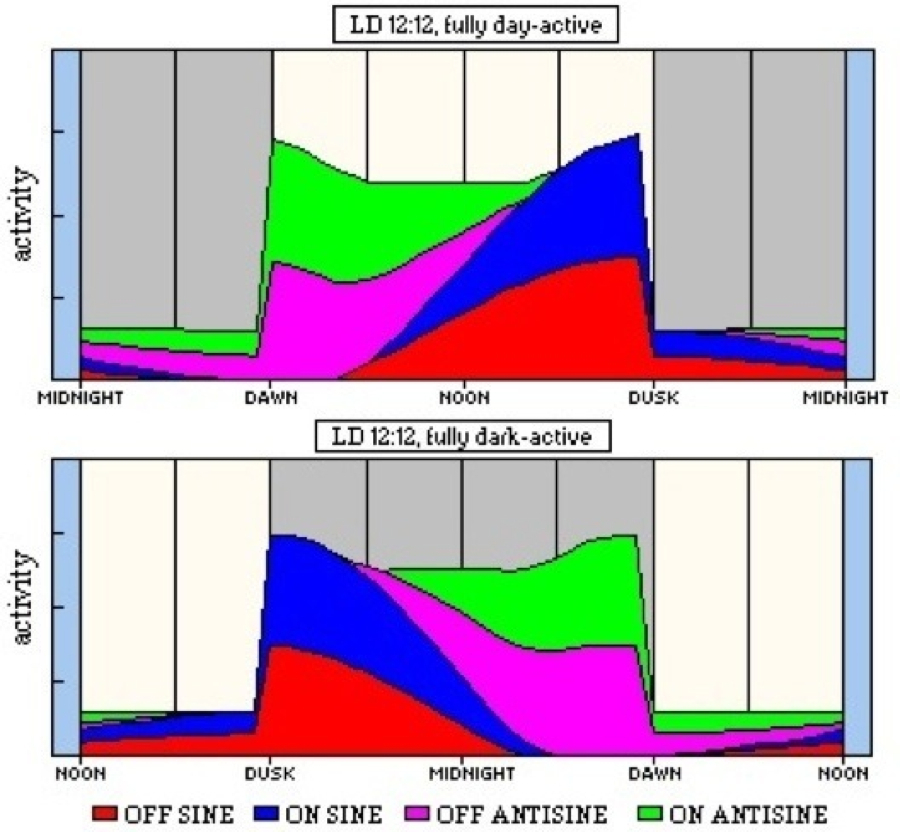
Figure 51 shows how summative effects might better explain the activity
patterns shown in apparently unimodal day-active or night-active
species. Support for this interpretation comes from reports of
ostensibly fully day-active insects, such as the blowfly, Lucilia
cuprina, where activity in LD 12:12 drops around noon (Smith, 1983).
Figure 51

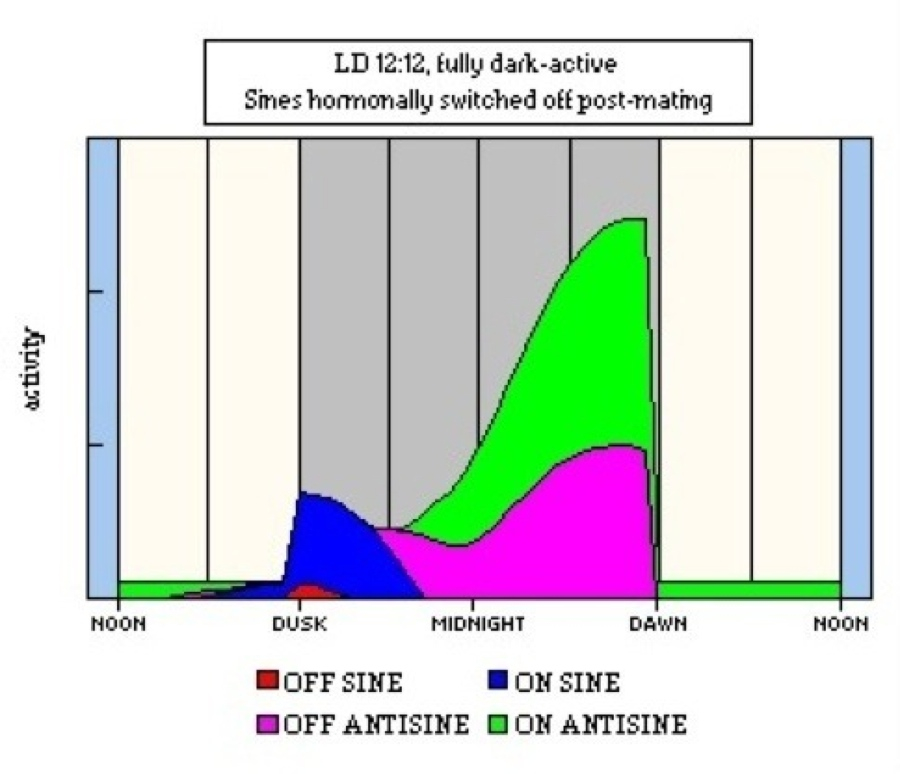
Another phenomenon that might be better explained by a summative effect is the change in activity timing that follows insemination in some mosquito species. In this, the flight of virgin females is timed to coincide with swarming males (usually very tightly timed around sunset) but mated females display a middle to late night pattern associated with host-seeking (Jones & Gubbins, 1979). The threshold for the SINE clocks may be shifted upwards by the hormonal factors introduced on insemination and, thus, most dusk or early night activity is inhibited. A similar threshold effect could explain the variation between groups of workers of the wood ant, Formica rufa, which are either night- or day-active (North, 1987). Figure 52 shows a model of such suppression of SINE clocks.
Figure 52
(j) Circadian periodicity
(i) Theory and determination
Broom (1980) gave the description that - "periodicity is a periodic variation within a time series", and he used correlograms (plots of autocorrelation coefficients against increasing lag) calculated from the time series of n activity counts for lags of < n/2. Activity was sampled at 30 min intervals and later at 5 min intervals. When n = 284, lags of 24, 48 and 96 were used; and when n = 1364, lags of 96, 288 and 576 were used. He used a c²/v test for the dominant peak on spectrograms (plots of spectral intensity), and compared actual spectrograms with artificially produced sine waves. Friesen et al. (1993) referred to normalised circadian hours. The concept in this paper, however, is based on the fact that there is no such thing as a "normalised" circadian hour. In insects, at least, each day consists of a period of dark and a period of light, and the clocks move at a different speed in dark and light (Dt and Lt).
(ii) Periodicity in mosquitoes
First, mention needs to be made of the report by Haddow et al. (1961) of a lack of rhythmicity in the oviposition cycle of Ae. aegypti when kept in LL, especially as this has been cited by Saunders (1982) and by Hong & Saunders (1994). When the results were re-appraised in the light of the flight activity patterns, the lack of observed periodicity turned out to be due to the observation method (which was to check for oviposition only every four hours), coupled with the authors' examination of results only for 24h periodicity (Taylor, 1969a). The fact was that the oviposition results actually did show LLt = 26h.
Anopheles gambiae showed a rhythm in DD with t @ 23h but no obvious rhythm could be found in LL (Jones et al., 1967).
Rowland (1989) with An. stephensi found evidence that the DDt was a little more than 24h, this might be due to the N peak being the fundamental expression of the clocks and to virgins having a physiological gating to match male swarming. Earlier, Jones (1974) had found that if the first cycle was omitted (as the first peak of activity in DD came some 25h after the final light-off) the rhythm settled with a DDt @ 23.7h. This species is almost completely inhibited by light and so LL periods were not observable.
Culex pipiens pallens (Kasai & Chiba, 1987) has been found to be bimodal with E and M peaks, in DD the cycle shortened and in LL there was no apparent rhythm. After ablation of the optic lobes a rhythm was seen in LL, however, with t thought to be about 15h. As with Cx. p. molestus (Chiba & Tomioka, 1992), it seems likely to me that the actual LLt @ 30h but this was not realised by the researchers. With another member of the Cx. pipiens complex, Cx. p. quinquefasciatus, Jones (1976), taking the E peak as his reference point, found that the length of the first cycle in DD appeared to vary cyclically as the final light period was extended. He felt that this related to the time of light-off in relation to the phase of the rhythm in LL but this seems to have been the limit of his consideration of the significance of period difference between DD and LL.
Nayar & Sauermann (1971) studied Aedes taeniorhynchus, which they reported as being night-active, with E and M peaks plus low level activity throughout the night. They found DDt @ 24h or less, but reported LLt as being much less than 24h. Again, perhaps the real situation is that LLt was similar to that in Cx. p. molestus and it was not realised that LLt in fact was around 30h, i.e. with the bimodal peaks appearing to give t @ 15h.
(iii) Periodicity in other insects
As with several of the mosquito species, a problem encountered in many circadian rhythm studies of other insects is the lack of activity in LL, in the case of dark-active species, and an apparent lack of rhythmicity in light-active species. Hong & Saunders (1994) have explored the effects of constant light (LL) on activity in the blowfly, Calliphora vicina, and reviewed other findings for insects exposed to DD, or LL at varying levels of light intensity. Overall, the findings (also summarised by Saunders, 1982) are that insects tend to show a longer t in LL than in DD. That is if a rhythm can be detected in LL, in C. vicina, for instance, light above about 21 lux led to apparent arrhythmia, and t increased with increasing light intensity up to that level (i.e. from an average of t = 23.7h in DD to an average of t = 24.6h in maximum LL).
In tsetse flies, which have a limited lifespan, a DDt of almost exactly 23h seems to be consistent for the several species which have been studied, e.g. G. longipennis, G. morsitans (Kyorku & Brady, 1994 et ante.). In LL the rhythm either disappears or is swamped under a level of near continuous activity for the three days or so before death occurs.
The fruit fly, Dacus tryoni, held in continuous low light shows a rhythm of mating which persists for at least four days with t @ 28h (Tyschen, 1978).
An Australian sheep blowfly, Lucilia cuprina, showed clear circadian rhythmicity for some five days in DD, t = 22.2h, and in LL of 1 lux or less, with a slightly higher period, t = 22.32h (Smith, 1983). In LL above 1 lux there were high levels of activity and Smith reported that there was no clear rhythm. Some of his individual examples, however, do seem to show weak periodicity with LLt @ 26h, which perhaps was not realised because the periodograms also showed a weak peak at around 13h. The origin of the blowflies was near Canberra, latitude 35°S, maximum L = 14.5h.
Honeybees, Apis mellifera ligustica, from Texas, 30°N, maximum L = 14h, have a DDt @ 23h in DD (Moore & Rankin, 1993).
Stengl & Homberg (1994) examined various neurological phenomena in a cockroach, Leucophaea maderae, and found a normal DDt = 23.5h. Damaging the optic stalks led to arrhythmia for several days but the rhythm reappeared with almost the same t as before or slightly shorter.
Honegger (1981) reported interesting results for singing activity in the cricket, Gryllus campestris, a species which has both day-active and night-active individuals. Both types showed rhythmic singing in LL, with t @ 26h (a German population, origin about 50°N latitude). Only the dark-active individuals would sing in DD and then t @ 23-24h.
An even more exceptional, perhaps unusually exceptional, example of changing periodicity and complex behaviour was described from experiments with the New Zealand weta, Hemideina thoracica, which is dark-active and long-lived in captivity. The DDt was typically close to or < 24h for the first 5-10 days, thereafter most individuals showed lengthening periods to t = 25-26h. Christensen & Lewis (1982) considered the first 5-10 days to be an after-effect of natural entrainment, and just an example of entrainment after-effects. Nevertheless, in dim LL (0.1 lux) t = 27.7h and was consistently longer than DDt. Hemideina also showed some splitting of a normally overtly unimodal pattern after some 50 days in DD, with re-uniting of the pattern at around 100 days.
An Australian field cricket, Teleogryllus commodus, studied by Wiedenmann (1983), also showed a tendency for the pattern to split into two activity components per cycle. Later, Wiedenmann (1988) gave the LLt > 24h and DDt < 24h; his graphs indicate that the LLt @ 26h. Rence et al. (1988) found that there was an increase in t as light increased from DD to a threshold, with t @ 23.5h in DD, and t @ 24h when light 0.00025 lux, above 0.01 lux a maximum t of 24h 50min was recorded. The colony used was no more than 5-6 generations from field collection at Wollongong, Australia, about 34°S, maximum L about 14.5h.
Although they studied a day-active insect, the Onion fly Delia antiqua, Watari & Arai (1997) recorded activity in DD but not LL. The mean DDt was 22.7 ± 0.64 for the first 10 days following rearing in LL and then slowed somewhat to 23.8 ± 1.05 by day 20. In DD following LD 12:12, the mean DDt was 22.2 at 10 days and 23.1 at 20 days. The overall level of activity, however, also declined with age. Interestingly the lengthening DDt with age was paralleled by the main E' peak falling progressively later - across the period 3 days post-eclosion to 30 days post-eclosion in LD 10:14 the peak moved from 4 h before light-off to 2 h before light-off.
(iv) Period mutants
The search for the clock at a biochemical level is gaining both momentum and, apparently, success at the present time (Crosthwaite et al., 1995; Goldbeter, 1995; Zeng et al., 1996). Much of the progress has stemmed from studies of locomotor activity of the fruit fly, Drosophila melanogaster, which for is long known to have so-called "Period" mutants. Saunders et al. (1994) list these according to period lengths displayed by the mutants in DD. These are a wild type with t @ 24h, perS with t = 19.7h, perL with t @ 29h, and perO which is arrhythmic. A further mutant perT with t @ 16h is mentioned by Goldbeter (1995).
With the realisation that, in the great majority of reported insects, Dt and Lt differ, and Dt < 24h and Lt > 24h, it is tempting to wonder if the per mutants actually are mutants in which the (presumed) photosensitive factors leading to Dt and Lt are singly present, rather than in the normal combination which acts to give the routinely observed LD 12:12 activity patterns.
The new work by Zeng et al. (1996) has models of how a timeless (tim) protein, which is rapidly degraded by light, is a stoichiometric partner of the period protein encoded by the per gene. The existence of intraspecific latitudinal variation in critical daylength is a well-known phenomenon (for instance, in the mosquito Aedes atropalpus, Beach 1978). Thus, it seems reasonable to suspect that a widely distributed species, such as D. melanogaster, will have such variants. The report of a very short t in perT (Goldbeter, 1995) might be the result of observation of a bimodal pattern; in which case the true t would be t = 32h, which is not unlikely given the range of period lengths found in mosquitoes, rather than the reported 16h.
Most recently, Tomioka et al. (1997) have reported findings on the effect of life cycles given during development on t of period mutants of D. melanogaster. The mutants used were the wild type "Canton-S", perS and perL1. Activity offsets, described as the "most reliable phase indicator", were used as the reference points to determine t. First, what is not obvious from the previous reports, was the recognition that the wild and perS insects were day-active, both strains having clear bimodal activity with M and E' peaks, E' being the higher of the two. On the other hand, perL1 was almost completely inhibited by light, and did not entrain in LD 4:20. In LD 8:16 to LD 16:8 most activity was middle-night, while in LD 20:4 the activity split into E and M'. When the LD regimes were changed to DD, the wild and perS types retained the bimodal rhythm, whilst perL1 displayed persistence of the peaks of the prior LD regime. In flies reared in DD a rhythm was observed, although no entrainment was obvious other than a time of weak red light while the flies were transferred from the rearing cages to the observational chambers. The determined t lengths were wild 24.2 ± 0.04 h, perS 18.7 ± 0.04 h and perL1 30.2 ± 0.19 h. In LL reportedly all three strains were arrhythmic; the c² periodograms, however, do show weak blips at around 24h in the wild and perS strains and, more markedly at about 26h in perL1. In all three strains the t lengths were longer for insects reared in DD than those reared in LL. Similarly t was longer in all strains when low but non-inhibitory levels of LL were used. An alternative interpretation of their results seems a possibility if full consideration was given to the effect of multiple peaking, which is obvious from their results, rather than the perhaps misleading use of activity "offsets" and "onsets". Very significant, however, may be their conclusion "that the effect of light is not constant but varies in non linear mode with the length of photoperiod".
Why so little has been made of the fact that perL strains are dark-active really is surprising. In the case of mosquitoes, it is unlikely that so little would be made of the difference between Cx.p. molestus and Cx.p.pipiens, which are similarly light-active and dark-active respectively. The mosquitoes are regarded as sibling species, difficult to tell apart by morphological methods and one has to question why the D. melanogaster strains are regarded as mutants and not as sibling species.
|
©1998, 2010 - Brian Taylor CBiol FSB FRES 11, Grazingfield, Wilford, Nottingham, NG11 7FN, U.K. Comments to dr.b.taylor@ntlworld.com |